

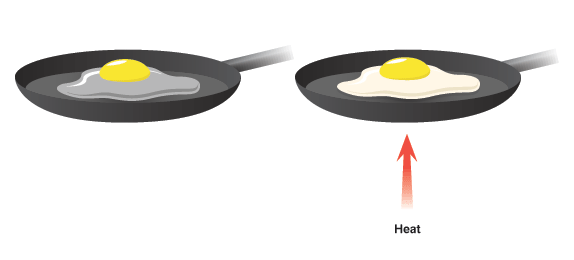
Sometimes, unripe fruits and vegetables have better health benefits than ripe fruits and vegetables. In the above image, the extreme left green – colored banana is the raw banana and the extreme right yellow banana with brown spots indicates ripened banana.Įvery so often, we hear that raw fruits and vegetables have different advantages as well as disadvantages from ripened fruits and vegetables. Chiefly, ethylene gas is responsible for ripening of fruits and vegetables and is often referred to as the ‘Food Ripening Hormone.’ Ripening of Banana Though the ripening of fruits and vegetables is not an entirely chemical process, it is a bio-chemical change.Īs a bio-chemical change, numerous enzymes, genes, and acids work together, breaking and making chemical bonds to alter the fruit and vegetable from being raw to ripe. The ripening process of any fruit or vegetable is considered as an irreversible chemical change example as once the fruit is ripened, it cannot be reversed as a raw fruit. Read more about Chemical Change Examples The Process of Ripening of Fruits and Vegetables Similarly, in the above chemical process, the reaction between carbon dioxide and water cannot create fuel and oxygen. Cake cannot be transformed back into flour, egg, sugar, etc. Once the wood turns to ashes, the ashes cannot be turned into wood again likewise, once the cake is baked we cannot un-bake the cake. The direction of the arrow is only in one way, which coveys that the reaction is irreversible.Ĭombustion of fuel is as simple an example as burning of wood or baking a cake. In this type of reaction, the fuel combines with oxygen in the air and emits products such as carbon dioxide and water vapor.įuel(C xH y)+Oxygen(O 2)→Carbon Dioxide(CO 2)+Water(H 2O) In this section, we’ll study in detail about some of the irreversible chemical change examples.Ĭombustion of fuel is an irreversible chemical change example. Properties like boiling point, melting point, molecular mass, color, odor, etc., change. When an irreversible chemical change occurs, internal properties of material change. Burning of fire-crackers, combustion, rusting, leaves changing color, decomposition of food are also some irreversible chemical change examples that we observe frequently.

Irreversible chemical change examples include much day- to- day work like cooking, eating, digesting, etc. Changes that occur only in one direction or changes that are permanent are known as irreversible chemical changes.


 0 kommentar(er)
0 kommentar(er)
